A flammable gas will spontaneously burn if the temeprature reaches a temperature known as the 'ignition point'. This temperature gives the gas molecules enough energy to break the bonds between atoms that keep molecules together. When these bods are broken, new bonds can be formed which may result in a net release of energy.
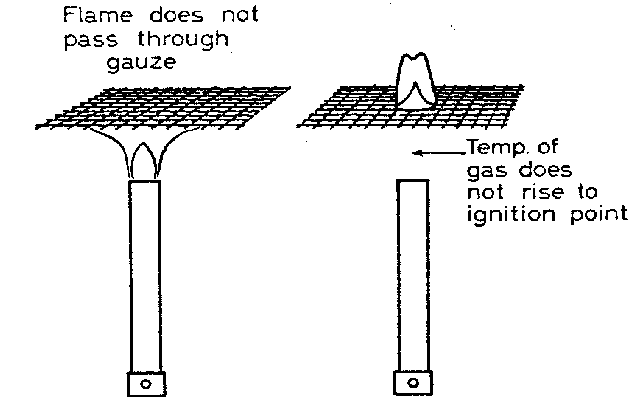
A good conductor can rapidy transfer and redistribute heat. The effect of this can be to reduce the temperature of a gas to a temperature below the combustion point. Suppose for example that we light a bunsen burner and put it under a wire gauze. The flame will not pass through the gauze because heat is rapidly conducted so that the gas above the gauze is at a temperature below the ignition point.
The gas is turned off, the gauze allowed to cool and the gas switched on again. The gas passing through the gauze is lit. The gas below the gauze remains unlit, because heat is conducted away rapidly so the gas below the gauze does not reach the temperature required for ignition.