The rate at which a gas spreads out depends on the speed![]() of the molecules of the gas, and since the kinetic energy of the molecules depends on the speed via the equation
of the molecules of the gas, and since the kinetic energy of the molecules depends on the speed via the equation![]() the rate of diffusion depends on the kinetic energy.
the rate of diffusion depends on the kinetic energy.
We can't measure directly the speed of the molecules of a gas, or the kinetic energy. Fortunately, if the gas behaves as an ideal gas, which most gases do at normal temperatures and pressures, the average kinetic energy of the gas molecules is directly proportional to the temperature so we can write![]() where K is a constant of proportionality, hence
where K is a constant of proportionality, hence![]() so that the average speed of the gas molecules is proportional to the temperature (the proportionality is not exact, even assuming an ideal gas).
so that the average speed of the gas molecules is proportional to the temperature (the proportionality is not exact, even assuming an ideal gas).
When a gas diffuses, the gas molecules spread out to occupy volume, and this is obviously proportional to the speed of the molecules, hence to the square root of the temperature.
At a given temperature, all the molecules of an ideal gas have the same kinetic energy. The kinetic energy is independent of the molecule – its mass, shape or size. The kinetic energy depends only on the temperature, so that gas molecules of different mass have the same kinetic energy. If we have two species of atom in a gas, of types 1 and 2, then
the average kinetic energy of species 1 is![]()
the average kinetic energy of species 1 is![]()
These are equal at the same temperature so![]()
We can simplify this expression and rearrange to give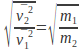 so that the rate of diffusion of a gas is inversely proportional to the square root of the mass of the gas molecules.
so that the rate of diffusion of a gas is inversely proportional to the square root of the mass of the gas molecules.
