There occur in nature about 300 nuclei, mostly representing isotopes of elements containing from one to 94 protons. Some 2,200 additional nuclei have been made artificially. Heavier nuclei become increasing difficult to make because the repulsive force between the positively charged protons increases faster than the attractive strong nuclear forces that hold the nucleons (protons and neutrons) together. The large electrostatic forces cause heavy nuclei to decay with the emission of alpha particles or spontaneous fission.
The nucleus has a shell structure. Electron around the nucleus occupy shells, and the atom becomes less reactive when these shells become full, meaning more stable, longer lived nuclei. It is thought that when shells in the nucleus become full , the nucleus will become more stable against decay, leading to the formation of 'islands of stability', as shown in the diagram below. A nucleus with a completely filled shell of either protons or neutrons is said to be magic because it is relatively more stable than nuclei with partially filled shells.. Most magic nuclei are spherical, but some can reduce their energy and become more stable, by rearranging their protons and neutrons into deformed shells accommodating different numbers of nucleons. The closing of these deformed shells leads to deformed magic numbers, with higher binding energy per nucleon, and more stable nuclei.
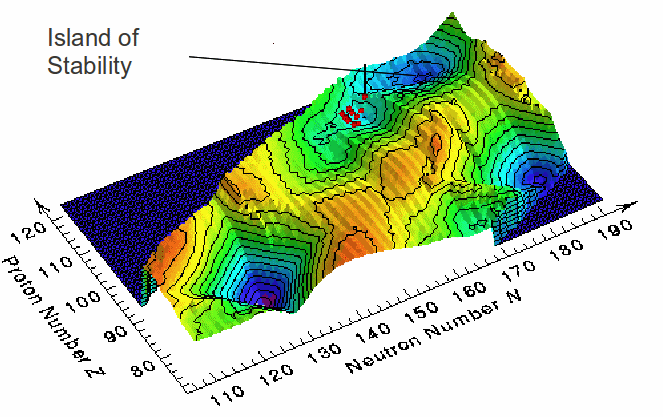
Calculations predict a that the next spherical proton and neutron magic numbers are 114 and 184 respectively, and deformed proton and neutron magic numbers at 110 and 162 respectively.
