The decay constant![]() appears in the equations
appears in the equations
![]()
![]() (1)
(1)
where![]() or
or![]() is the number of undecayed atoms at any time
is the number of undecayed atoms at any time![]()
![]() is the activity or number of decays in one second and
is the activity or number of decays in one second and![]() is the number of undecayed atoms at time
is the number of undecayed atoms at time![]()
Many elementary textbooks explain that![]() is the probability of a single atom decaying in one second. This is false, and equation (1) is also misleading – it is only theoretically true in the limit of infinite half life,
is the probability of a single atom decaying in one second. This is false, and equation (1) is also misleading – it is only theoretically true in the limit of infinite half life,![]()
The actual meaning of![]() is: in each time period
is: in each time period![]() a fraction
a fraction![]() of the undecayed atoms at the start of the time period will remain undecayed at the end of the period. We can rewrite (1) with
of the undecayed atoms at the start of the time period will remain undecayed at the end of the period. We can rewrite (1) with![]() to become
to become![]() with dt tending to 0 so that
with dt tending to 0 so that![]() is the instantaneous rate of change of
is the instantaneous rate of change of![]() The minus sign is necessary because
The minus sign is necessary because![]() is decreasing so
is decreasing so![]() is negative.
is negative.
To see why the distinction is important, consider an isotope with a very short half life, say![]()
Then from the equation relating![]() to
to![]()
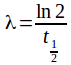 we have
we have![]() but no probability greater than one is possible.
but no probability greater than one is possible.
