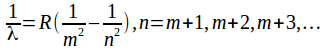Hydrogen is the simplest atom, and during the development of quantum mechanics, attracted the most attention. Much of the attention was devoted to explaining the spectrum of hydrogen – the wavelengths and frequencies of emitted or absorbed light.
The spectrum of hydrogen falls into distinct series. When a hydrogen atom emits or absorbs radiation, the electron jumps from one energy level to another. The lower of these energy levels determines the series of the spectrum in which the emitted or absorbed radiation falls.
The wavelengths of the radiation in each group are described by an equation.
Wavelengths in the Lyman series are given by the formula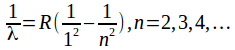
![]() is a constant called the Rydberg constant.
is a constant called the Rydberg constant.
Wavelengths in the Balmer series are given by the formula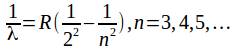
Wavelengths in the Paschen series are given by the formula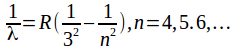
Wavelengths in the Brackett series are given by the formula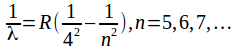
Wavelengths in the Pfund series are given by the formula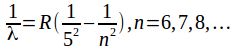
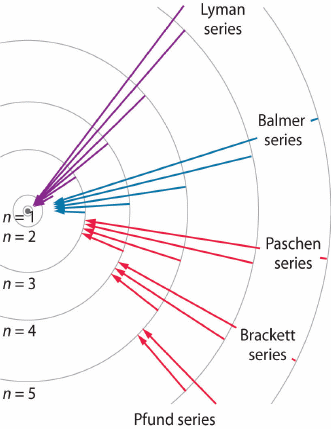
Other series exist. The general formula is given by