The root mean square (rms) speed is a measure of the average speed of a gas. It is not the same as the mean speed or mean velocity – in fact the gas molecules inside a closed container move as much in one direction as another, so the mean velocity is zero. The root mean square speed is not zero because all the velocities are squared first, added and then the square root is found.
Suppose for example that a gas consists of only 5 molecules with speeds 200, 300, 400, 500 and 600 m/s respectively.
The mean speed is![]()
The root mean speed is sqrt![]()
The root mean square speed of a gas is always greater than the mean speed. This is because![]() is related to the variance or spread of a set of data. The variance is always greater than zero if the data takes more than one value.
is related to the variance or spread of a set of data. The variance is always greater than zero if the data takes more than one value.
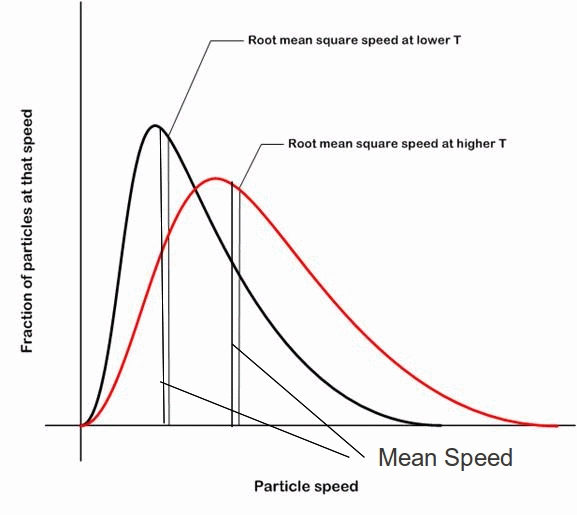
The mean and root mean square speed both increase with temperature. The rms speed is a measure of the average kinetic energy of the molecules of a gas because![]() All the molecule in a gas have the same average kinetic energy because we can also write
All the molecule in a gas have the same average kinetic energy because we can also write![]() where
where![]() is Boltzmann's constant and
is Boltzmann's constant and![]() is the absolute temperature. Hence rms speed decreases with increasing mass.
is the absolute temperature. Hence rms speed decreases with increasing mass.
