The model of ideal gases treats gases as a collection of hard non - interacting particles of zero volume. These gases obey the ideal gas laws – Charles's Law,![]() the Pressure Law,
the Pressure Law,![]() and Boyle's Law,
and Boyle's Law,![]() collectively summarised in the ideal gas equation, often stated as
collectively summarised in the ideal gas equation, often stated as![]()
where
p=pressure, V= volume, T=temperature, n=number of mols and R=the Universal Gas Constant. 8.314 J/K/mol.
In fact no gas is ideal under extreme (high) pressure and low temperature. This is perhaps best shown by plotting curves of pV against p and p against V for a constant mass of gas as shown below for carbon dioxide.
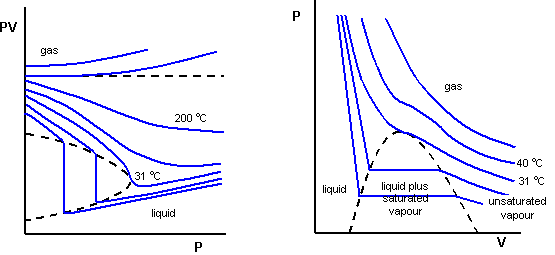
For Boyles Law to be obeyed, we would expect pV to be constant. The graph on the left shows deviations from Boyles law increase at lower temperatures. Both graphs show something peculiar happening on the 31 degrees Celsius isotherm. At this temperature, called the critical temperature, for a particular pressure, the liquid and gas phases are indistinguishable. Above this temperature, carbon dioxide could not be liquified by pressure alone, while below this temperature, a decrease in volume would result in gas condensing into liquid at constant pressure. This combination of pressure, temperature and volume is called the critical point.
Some critical temperatures are shown in the table below.
|
Substance |
Critical Temperature (Degrees Celsius) |
|
Helium |
-268 |
|
Hydrogen |
-240 |
|
Nitrogen |
-147 |
|
Air |
-140 |
|
Oxygen |
-118 |
|
Carbon Dioxide |
30.9 |
|
Chlorine |
146 |
|
Water |
374 |
