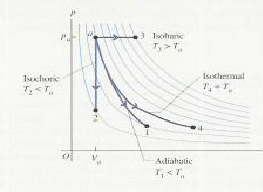
There are for types of thermodynamic processes that gases may undergo. These are:
-
Adiabatic. No heat transfer occurs. This mean that
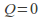 in the equation
in the equation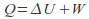 so ifthe gas expands and does work –
so ifthe gas expands and does work –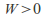 – the gas cools down -
– the gas cools down -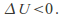 Conversely if the gas contracts –
Conversely if the gas contracts –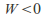 – the gas heats up -
– the gas heats up -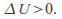
The gas may be insulated, or the adiabatic process may occur so quikly that there is insufficient time for any heat transfer. The compression stroke in an internal combustion engine is approximately adiabatic. The temperature rises as the air fuel mixture is compressed. The expansion of the burnt fuel during the power stroke is also an approximately adiabatic expansion with a drop in temperature.
-
Isochoric. The volume is kept constant. The gas does no work on it's surroundings.Any energy supplied to the gas becomes internal heat energy because W=0 in the equaton
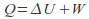 Note however there are types of work that do not involve a volume change. For example we can do work on a fluid by stirring it.
Note however there are types of work that do not involve a volume change. For example we can do work on a fluid by stirring it. -
Isobaric. The pressure is kept constant. In this case
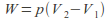 so
so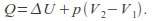 Any process that takes place in the open is effectively isobaric because the pressure is atmospheric pressure.
Any process that takes place in the open is effectively isobaric because the pressure is atmospheric pressure. -
Isothermal. The temperature is kept constant. Heat must be allowed to flow into and out of the system, and the process must take place slowly enough for thermal equilibrium is maintained. In general none of
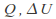 or
or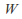 is zero for an isothermal process. The internal energy of an ideal gas depends only on the temperature so
is zero for an isothermal process. The internal energy of an ideal gas depends only on the temperature so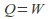 and heat supplied to the system becomes work done by the system.
and heat supplied to the system becomes work done by the system.
The different types of process are shown on the![]() diagram below.
diagram below.