The continuous flow calorimeter, shown below, has the advantage that we do not need to know the heat capacity of the calorimeter itself to find the specific heat capacity of the liquid in question.
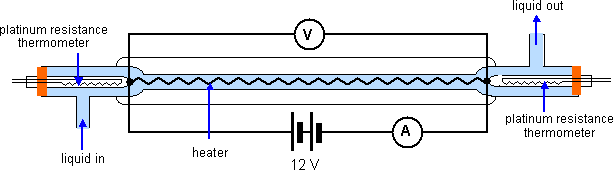
Liquid flows in from the bottom left at a constant rate past a platinum resistance thermometer, past a heater (insulated inside a vauum jacket) and then past a second platinum resistance thermometer, exiting the apparatus up to the right. The temperatures![]() and
and![]() at the first and second thermometers are recorded. When these temperature have reached stable values, the temperatures and the water flow rates are recorded.
at the first and second thermometers are recorded. When these temperature have reached stable values, the temperatures and the water flow rates are recorded.
The electrical energy supplied is given by![]() where
where![]() is the current recorded on the Ammeter and
is the current recorded on the Ammeter and![]() is the Voltage recorded on the Voltmeter over a time
is the Voltage recorded on the Voltmeter over a time![]()
Carrying out the experiment once gives![]() (1)
(1)
where![]() is the mass of liquid which flows in a time
is the mass of liquid which flows in a time![]() and
and![]() is the heat capacity of the calorimeter.
is the heat capacity of the calorimeter.
Adjusting the water flow rate and voltage, so that the inflow and outflow temperatures remain the same gives a second set of results resulting in the equation
![]() (2)
(2)
(2)-(1) gives![]()
