Van der Waals forces are forces between atoms or molecules that arise because of fluctuations in the distribution of charge on atoms or molecules. Charges on atoms can randomly distribute themselves so that one side is negatively charged and the other end is positively charged.
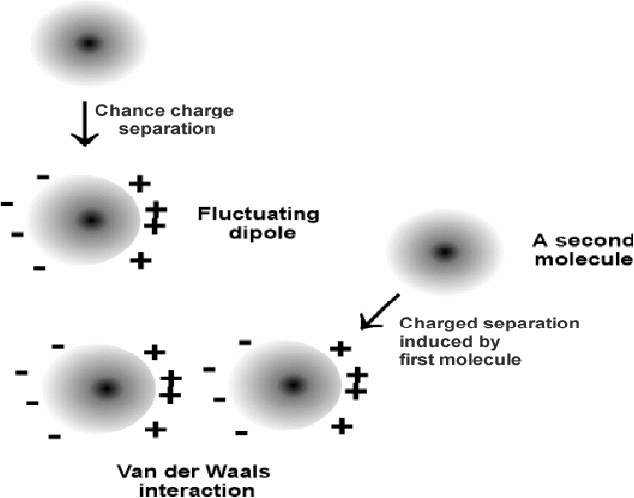
This can the induced dipoles of opposite sense on surrounding atoms or molecules. Dipoles of opposite polarity attract each other. The net effect is that overall the atoms of a gas or liquid attract each other because of random fluctuations in the distribution of charge on the atom or molecule.
Van der Waals forces are very weak, but play important roles in evaporation and condensation.
