If the temperature of a liquid is fixed and evaporation takes place in a closed system, and there is an excess of liquid, then a point will come at which there will be no net evaporation. Liquid molecules will leave the liquid, as vapour but they will also return to the liquid at the same rate overall. The vapour is saturated.
As the liquid heats up, the rate of evaporation of the liquid increases. At some point the rate at which molecules return to the liquid will again equal the rate at which they leave it, but the equilibrium will be at a higher saturated vapour pressure.
We may also explain the mechanism in this way. Consider a bubble in the liquid. The pressure within the bubble is the saturated vapour pressure at the temperature of the liquid, neglecting the pressure due to the water above the bubble. As the liquid heats up, more bubbles form in the liquid and will tend to rise because they are less dense than liquid, so the rate of evaporation increases with temperature.
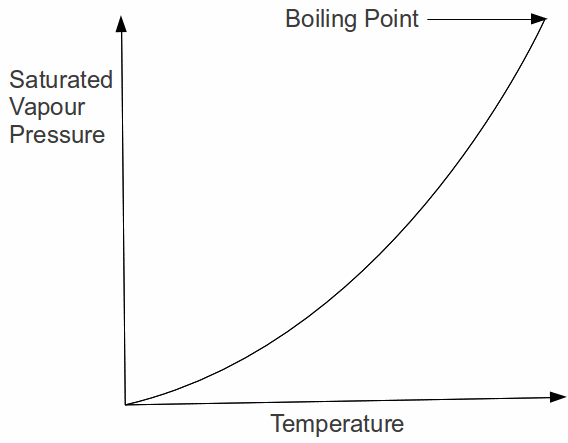
The last explanation provides insight into the boiling process. When the liquid boils, bubbles are formed within the liquid at a rapid rate because the liquid molecules have enough energy to break the transitory bonds between the liquid molecules. The bubbles rises to the surface and escape so the vapour pressure inside the bubble must be equal to the pressure above the liquid. We can say therefore: a liquid boils when it's saturated vapour pressure is equal to the external pressure. The variation of saturated vapour pressure for a typical liquid is shown below for a liquid from it's melting point at the origin up to it's boiling point.