The temperature of a material reflects the average total kinetic energy of its' particles, or the thermal energy stored in the material. Thermal energy is stored as kinetic energy in the random modes of translation in monatomic substances, and translations and rotations of polyatomic molecules in gases. Additionally, some thermal energy may be stored as the potential energy associated with higher-energy-modes of vibration, whenever they occur in interatomic bonds in any substance. Translation, rotation, and the two types of energy in vibration (kinetic and potential) represent the degrees of freedom of motion which classically contribute to the heat capacity of a thermodynamic system.
For quantum mechanical reasons, some of these degrees of freedom, being associated with higher energies, may not be available, or only partially available to store thermal energy, at a given temperature. As the temperature approaches absolute zero, the available degrees of freedom decreases and the specific heat capacity of a system approaches zero. This is illustrated below for three metals.
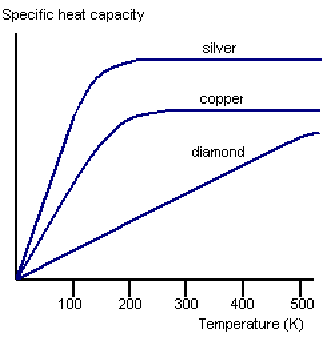
This implies that it impossible to reach a temperature of absolute zero, since an infinitesimally small amount of heat energy is sufficient to raise the temperature if the specific heat capacity is infinitesimally small.
