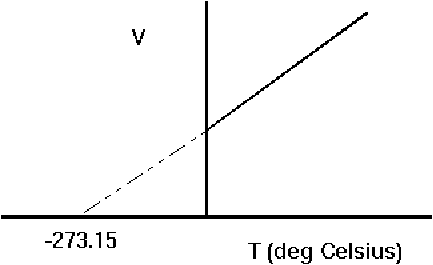
If volume - temperature or pressure – temperature graphs for a gas are plotted and extrapolated backwards they cut the temperature axis at -273.15 degrees Celsius, implying that at the this temperature the volume and pressure are zero.
Moreover we can plot the same graph for different gases and all the graphs will intersect the x - axis at the same point, suggesting a very fundamental temperature.
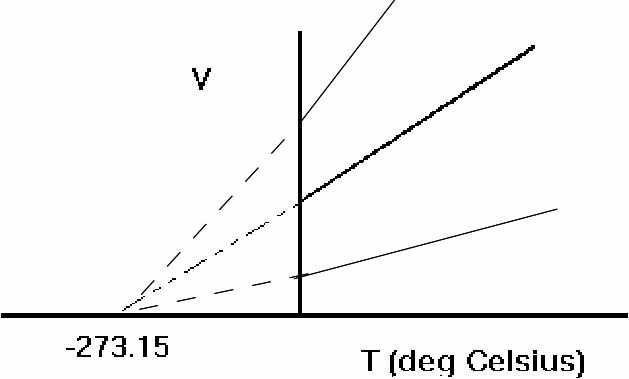
This suggests that -273 is the lowest attainable temperature or 'absolute zero'. This is only an assumption, since gases will liquify before this temperature is reached.
It is the absolute temperature![]() that appears in the ideal gas equation
that appears in the ideal gas equation![]() the pressure law
the pressure law![]() and Charles's Law,
and Charles's Law,![]() We obtain the absolute temperature, measured in Kelvins, K from the temperature in Celsius by adding 273.15.
We obtain the absolute temperature, measured in Kelvins, K from the temperature in Celsius by adding 273.15.
