You may already know that the boiling point of a liquid decreases with decreasing pressure. The boiling point of water at the top of Mount Everest is only about 40 °C. Conversely, the boiling point of water increases with increasing pressure. This fact may be used to cook food more quickly, saving time and energy. The cooking of food at high pressure may be done in a pressure cooker.
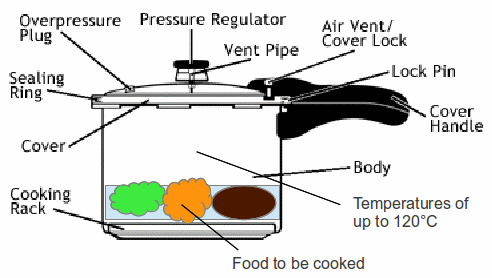
A modern pressure cooker is shown above. It consists of a heavy gauge aluminium saucepan and a lid weath a rubber seal. The lid is fitted with a valve which can be set to release steam at pressures varying from one and a third atmospheres to two atmospheres. Cooking time is reduced and energy is saved.
Pressures cookers are very useful at high altitude. In Quito, the capital of Ecuador, water boils at 90 ° C, but can be made to boil at the normal or higher temperature using a pressure cooker. This is necessary for some foods so that all bacteria are killed.
