Some liquids conduct electricity. Such liquids are called electrolytes. In order for a liquid to conduct electricity it must contain charged particles called ions, which are free to move in the liquid (electricity is basically just the flow of charge). Pure water is a poor electrolyte because it has very few ions, but can be made a much better electrolyte with the addition of a small amount of acid eg sulphuric acid. The acid introduces ions. Adding sulphuric acid to water produces![]() and
and ![]() ions.
ions.
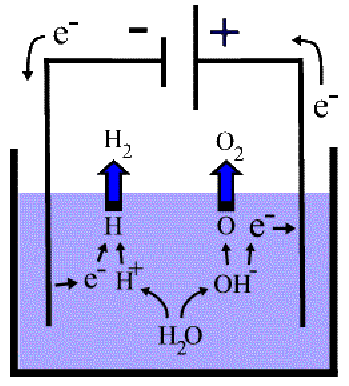
The molecular formula for water is H-2 O, so that in any amount of water there are twice as many hydrogen atoms as oxygen atoms. Because hydrogen and oxygen molecules both contain two atoms – they form![]() and
and![]() molecules – and equal numbers of atoms of any gas occupy the same volume at the same temperature, the volume of hydrogen liberated will be twice the volume of ocygen liberated.
molecules – and equal numbers of atoms of any gas occupy the same volume at the same temperature, the volume of hydrogen liberated will be twice the volume of ocygen liberated.
