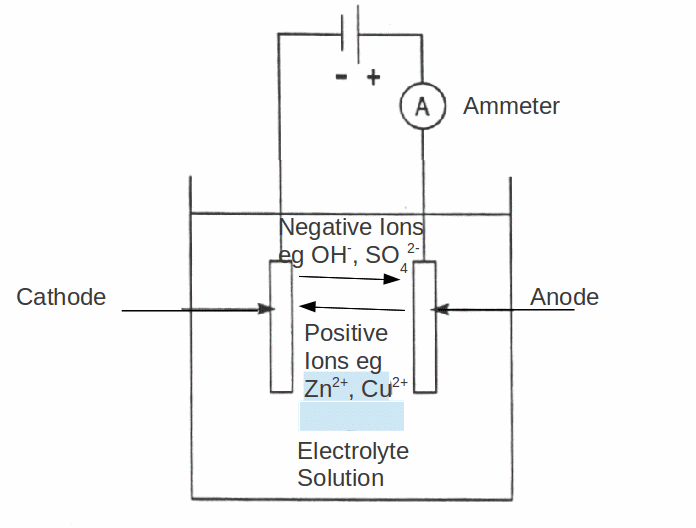
When a battery is connected between metal plates (electrodes) dipped in a solution, current may flow. One of the electrodes – that connected to the positive terminal of the battery will dissolve into the water, and the other electrode, that connected to the negative electrode, will gain mass – in fact the mass gained will be all metal. The electrode that dissolves is called the anode and the electrode that gains mass is called the cathode. The cathode having gained mass is said to have been electroplated.
Experiments show the mass of metal deposited is proportional both to the current and the time for which it is passed, In fact it is proportional to the charge passed, because![]()
Electroplating is widely used in industry. Jewellery may be gold plated this way and electrolysis is one method of purifying metal. It is the main method of purifying aluminium.
