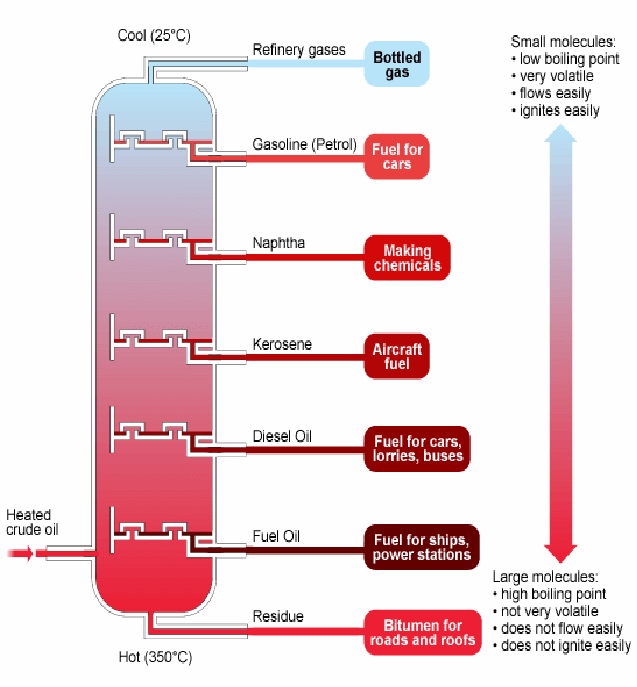
Fractional distillation is used to separate the constituents of crude oil into it's different useful components. The underlying principle is that the different components of crude oil boil (or evaporate) at different temperatures. Short chain hydrocarbons evaporate first. These are the smallest lightest molecules, and the forces between neighbouring molecules are weakest. There is a temperature gradient down the fractionating column. Methane and butane with low boiling points, form a gas at the top, while (dense) fuel oil, with a very high boiling point, forms boils only in the hottest region at the bottom. Each part can be drawn off at that part of the column where it forms a vapour.