The Earth is bombarded by electromagnetic radiation all the time. Some of it is very harmful – ultraviolet rays, X – Rays and Gamma Rays – but most of the harmful radiation is prevented by hitting the Earth's surface by the atmosphere. Ozone is a molecule in the Earth's atmosphere that absorbs radiation in the ultraviolet part of the electromagnetic spectrum. Most ozone in the Earth;s atmosphere is between 25 and 30 km above the Earth's surface, and this is called 'the ozone layer'.
When ultraviolet light strikes an oxygen (O 2 ) molecule, the molecule is split up into two oxygen atoms. One of these oxygen atoms may react with an oxygen molecule to form an ozone (O 3 ) molecule. Ozone also absorbs ultraviolet radiation, and in doing so splits to become an oxygen atom and an oxygen molecule. The free oxygen atom can combine with another oxygen atom to form another ozone molecule.
This cycle generally maintains a level of ozone in the ozone layer which prevents ultraviolet radiation from reaching the Earth's surface, protecting life on Earth.
The amount of ozone in the atmosphere is seasonal. In the 1970s scientists discovered a drop in the amount of ozone over the Antarctic with each passing spring, and a drop in the amount of ozone in the ozone layer over the rest of the world too.
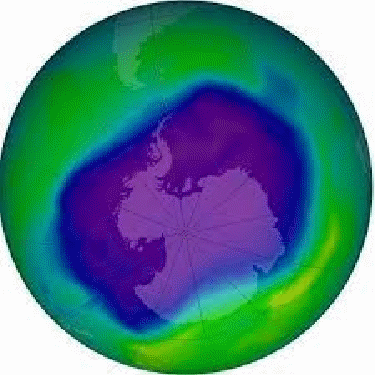
Scientists think the drop in the level of ozone was caused by the release ofd chemicals calld chlorofluorocarbons or CFCs. This is a chemical used in aerosols and refrigerants. In 1987 the Montreal Protocol was signed to cut down on the use of CFCs. This should allow the ozone layer to recover over time.
