The question of whether light was a particle or a wave is an old one. Newton thought light was made up of particles, Young that it was made up of waves. The argument seemed to be settled in favour of the wave theory until the photoelectric effect and the birth of quantum physics.
The photoelectric effect seemed to contradict that light could be a wave. If light were a wave, an electron close to the surface of a metal could gradually absorb energy and would eventually have enough energy to escape the surface of the metal. This would happen whatever the intensity or frequency of the light incident on the metal. The photoelectric effect clearly demonstrated that no electrons at all would be emitted if the frequency of the incident light were below a certain level, called the threshold frequency.
The photoelectric effect demanded an explanation that involved light not behaving as a wave. Einstein provided it. His explanation was
-
electrons near the surface of the metal need a certain minimum energy in order to escape the surface. This minimum is called the work function of the metal and is given the symbol
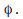
-
light arrives as packets of energy called photons.
-
the energy
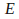 in each packet is described by the equation
in each packet is described by the equation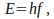 where
where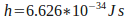 is a constant called 'Planck's constant', and
is a constant called 'Planck's constant', and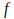 is the frequency of the light. The frequency of light needed to free electrons from the metal puts it in the ultraviolet range.
is the frequency of the light. The frequency of light needed to free electrons from the metal puts it in the ultraviolet range. -
different electrons absorb different photons. If the energy of the photon is large enough, the electron will be given enough energy to leave the metal.
-
any excess of the energy absorbed by the electron (the photon energy) over the energy needed to leave the metal surafce (the work function, %phi) becomes kinetic energy of the electron.
-
if the energy of the photon is not enough to allow it to leave the metal, it will become kinetic energy of the electron inside the metal and will soon be distributed among many electrons through collisions.
-
the photoelectric effect is described by the equation
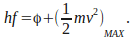 This is called the photoelectric equation.
This is called the photoelectric equation. -
the minimum frequency of radiation needed to produce photoelectrons is called the threshold frequency. Electrons will be produced, but when they are far away from the metal they will have no kinetic energy.
