Entropy is a property that expresses the disorder in a system, and increasing entropy means increasing disorder. It is linked to the number of possible states of the system, so that a system with more possible states has more entropy.
Consider a box split into halves. Initially all the particles are in the left hand side. It is more likely that there will be roughly equal numbers of particles in each compartment, because although each possible arrangement or state of particles is equally likely, there are more possible arrangements with roughly equal numbers in each half than with all particles in the left hand side.
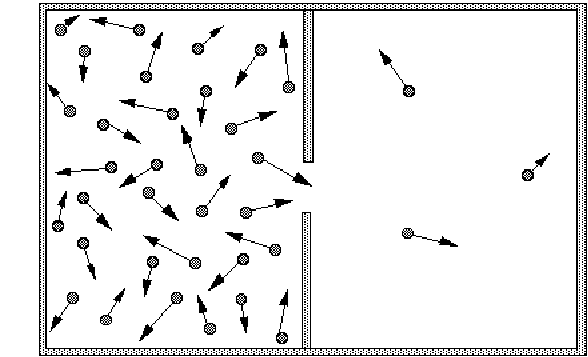
Because of this the entropy of the system with equal numbers in each half is greater than that with all particles on the left hand side.
In any random process, the entropy or amount of disorder will increase. There is in fact a relationship between the change in entropy![]() of a system, the heat
of a system, the heat![]() lost or gained by the system and the temperature
lost or gained by the system and the temperature![]() at which this loss or gain occurs:
at which this loss or gain occurs:![]()
When energy
![]()
The entropy change for the second body is![]()
The net entropy change is![]() since
since![]()
This shows that entropy increases when heat flows from a hot to a cold body.
The energy here has been degraded. It is harder to put to use. Ultimately the bodies will be in thermal equilibrium. Energy cannot be extracted from systems consisting of bodies in thermal equilibrium with each other.
