Evaporation is the process by which a liquid becomes a gas. Evaporation may take place at any temperature, but occurs most quickly when a liquid boils. Below the boiling point, the molecules in the surface of a liquid do not have enough kinetic energy on average to leave the liquid, but a fraction of molecules do and evaporation can still take place. Of course, it is typically the faster molecules that leave the liquid. The lower energy molecules remain as liquid, and higher energy molecules may leave escape the liquid but be pulled back, with even higher energy molecules able to escap altogether.
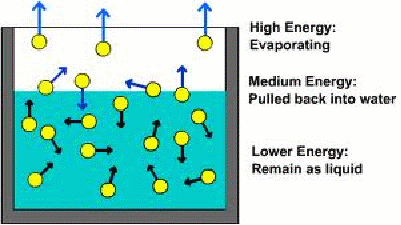
If the system is isolated then the most energetic molecules remove from the liquid whatever energy they have. The molecules left behind have less energy on average, so the rate of evaporation will decrease. In practice no system is ever isolated. The liquid will be at the ambient temperature usually, and energy may be given to a liquid from it's surrounding to replace that energy taken away by the liquid that has evaporated. The net effect is that in the absence of the amount of liquid being replenished by other means, all the liquid will eventually evaporate.
The rate at which evaporation takes place depends on:
-
The surface area of the liquid. Increased surface area means increased rate of evaporation.
-
The temperature og the liquid. Higher temperatures mean higher evaporation rates.
-
Air pressure. The evaporation rate increasing with decreasing atmospheric pressure.
-
The amount of vapour present in the air. The net rate of evaporation is a balance between vapour molecules rejoining the liquid and liquid molecules leaving. If there is a lot of vapour present, the rate at which molecules rejoin the liquid will increase and net evaporation will decrease.
-
A draught above the liquid surface will increase the evaporation rate by giving kinetic energy to molecules near the surface.
