In theory, if an object can be heated with no energy loss, then the increase in temperature depends on three factors:
-
the energy given to the object,
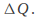
-
the mass m.
-
the substance from which the object is made.
In particular, the shape or colour of the object do not matter.
The heat capacity C of an object is the energy required to raise the temperature of the object by 1 K. Different objects and different samples of the same substance (having different masses) have different heat capacities. The specific heat capacity is the energy required to raise the temperature of 1 kg of a substance by 1 K.
![]()
![]()
Heat and specific heat capacities are often taken to be constant for a particular solid, liquid or gas, but this is only for ease of calculating. In fact as the temperature increases from absolute zero, on average so do the heat and specific heat capacities, depending on the state of a substance and on it's temperature. This is true in practice because as a material is heated so that the temperature is much higher than the surroundings, it loses energy to its' surroundings at a greater rate, but it is also true theoretically because of as a material is heated the number of ways in which it can absorb energy increases. This theoretical effect means more energy must be supplied to heat the substance by 1 K as the temperature increases, or that if a material is heated at a constant rate, the rate of increase of temperature decreases as the temperature increases.
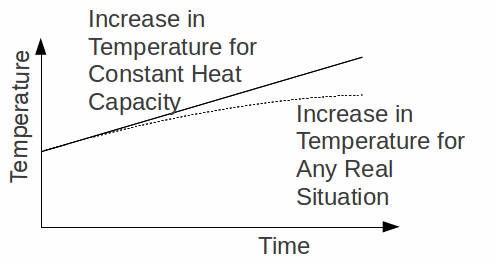
At the other end, it means that in practice the absolute zero of temperature is unreachable because an infinitesimally small amount of energy must be supplied to raise the temperature from absolute zero.
