There are three fundamental laws of thermodynamics. The first law is simply a statement of conservation of energy applied to the system. If an amount of thermal energy![]() is given to the system, either
is given to the system, either
-
The internal energy U of the system can increase by an amount
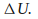
-
It can do an amount of work
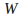 on its' surroundings.
on its' surroundings. -
It can do a combination of the above.
Since energy is conserved,![]()
The following must be noted:
-
Each of the quantities
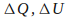 and
and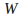 in the equation above is considered from the systems point of view.
in the equation above is considered from the systems point of view. -
If
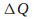 is positive, then the system gains thermal energy and if
is positive, then the system gains thermal energy and if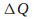 is negative the system loses thermal energy.
is negative the system loses thermal energy. -
If
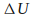 is positive, the internal energy of the system increases. If the system is a gas, then the temperature of the gas increases. Conversely if
is positive, the internal energy of the system increases. If the system is a gas, then the temperature of the gas increases. Conversely if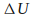 is negative the internal energy of the system is decreasing. If the system is a gas, the temperature of the gas is falling.
is negative the internal energy of the system is decreasing. If the system is a gas, the temperature of the gas is falling. -
If
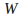 is positive then the system is doing work on the surroundings – a gas is expanding for example. If
is positive then the system is doing work on the surroundings – a gas is expanding for example. If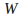 is negative the surroundings are doing work on the system. W is negative.
is negative the surroundings are doing work on the system. W is negative.
