If we make several plausible assumptions, the macroscopic properties of a gas can be expressed simply in terms of three gas laws. The assumptions are:
-
All collisions that take place between the molecules of a gas or between the gas molecules and the walls of the container are elastic, so the total kinetic energy of the gas is conserved. In addition the time of each collision is zero.
-
The volume the the actual gas molecules is small compared with the volume of the container. In fact calculations indicate the actual volume of the gas molecules is of the order of 1% of the volume of the container under normal conditions – room temperature and atmospheric pressure.
-
There are no forces between the gas molecules or between the gas molecules and the walls of the container.
-
The motion of the gas molecules is random, with no preferred direction. There is no bulk movement of the gas.
Under these conditions a gas will obey three simple laws.
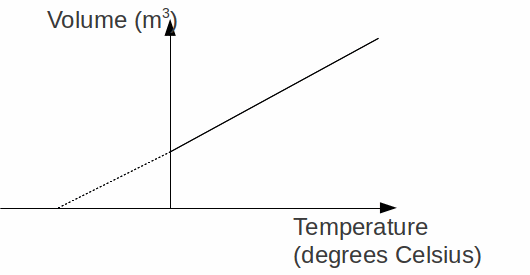
The Pressure Law: The pressure![]() of a fixed mass of gas increases linearly with temperature
of a fixed mass of gas increases linearly with temperature![]() at constant volume. This can be expressed as
at constant volume. This can be expressed as![]()

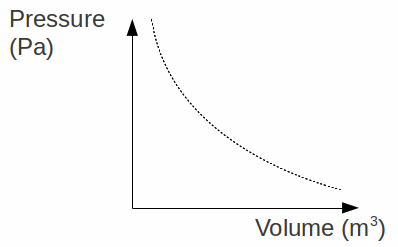
Boyle's Law: The pressure![]() of a fixed mass of gas is inversely proportional to the volume
of a fixed mass of gas is inversely proportional to the volume![]() at constant temperature. This can be expressed as
at constant temperature. This can be expressed as![]()