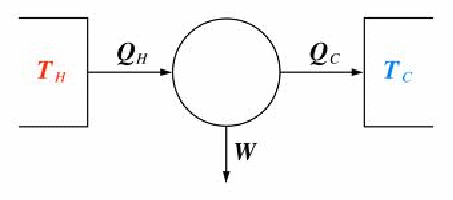
A heat engine is a device that uses a source of thermal energy to do work by converting heat into work. Internal combustion engines, steam engines and power station turbines are all heat engines. The ideal heat engine is shown below. It consists of a infinitely large hot reservoir at a temperature![]() and an infinitely large cold reservoir at a temperature
and an infinitely large cold reservoir at a temperature![]() Heat flows from the hot to the cold reservoir. Being infinitely large, the temperature of neither reservoir is changed as a result of heat flow. Some of the heat transferred can be made to do work.
Heat flows from the hot to the cold reservoir. Being infinitely large, the temperature of neither reservoir is changed as a result of heat flow. Some of the heat transferred can be made to do work.
The thermal efficiency of a heat engine is given as![]()
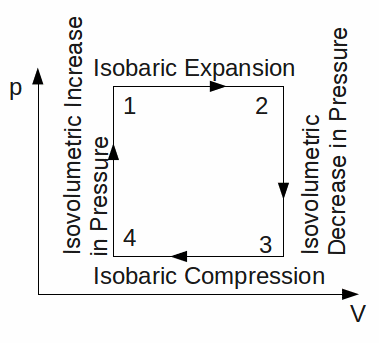
The cycle that results in the maximum possible efficiency is called the Carnot cycle.
