Ernest Rutherford, working at Cambridge, designed an experiment which gave illustrated the nature of the nucleus. He fired alpha particles - relatively heavy particles produced by radioactive decay - at a thin piece of gold foil. At that time many people thought the atom to be like a solid little billiard ball, solid throughout.
When alpha particles were fired at the foil, most of them passed through, as though they had passed through empty space. This showed that atoms were mostly empty space.
A few were slightly deflected, showing that they had approached the nucleus, and had been deflected by it.
A very few were deflected back, close to the foil, indicating a head on collision with the nuclear, and that the nucleus was positively charged.
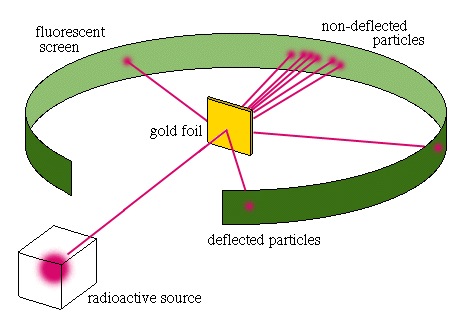
The size of the nucleus could be estimated from the relatively frequencies and degrees of deflection. The size of the nucleus was tiny, much smaller than the size of the atom, and resulted in the view that the nucleus, with most of the mass at the centre of the atom was tiny and positive, and that the electrons surrounded the nucleus, like an atom sized solar system.

