Joules experiments on work and heat transfer demonstrate that mechanical or electrical energy can be changed into internal energy to produce a rise in temperature. The apparatus for one of his experiments is shown below.
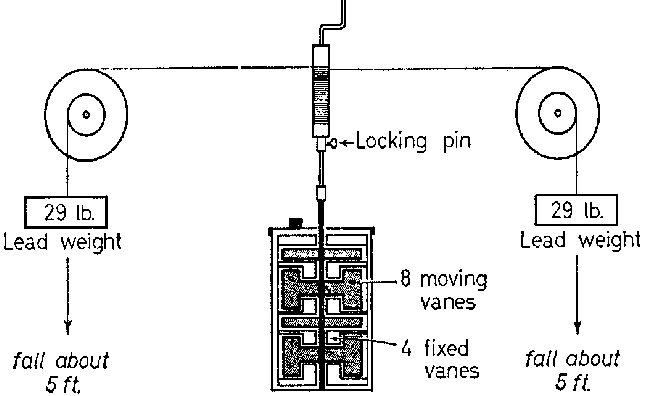
Two heavy lead weights are attached to string wound around the spindle of a paddle wheel free to rotate in a copper vessel containing water. It is important that to maximise the amount of motion which manifests as heat that the motion of the water is mall scale and random. To achieve this, there are fopur fixed vanes inside the vessel, then the work done by the paddle against the resistance of the water is transferred to internal energy of the water and copper.
The handle is wound repeatedly so that the weights are made to rise and fall several times. Joule was able to show that the total potential energy transferred by the weights as a result of rising and falling several times was equal to the energy of heat gained by the water.
