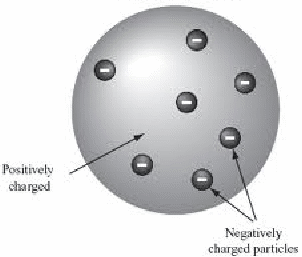
In the early twentieth century, the structure of the atom was unknown. One well thought of possible model – diagram below - described the atom a a 'plum pudding', with negatively charged electrons scattered in a uniformly positively charged nucleus.
Ernest Rutherford suggested firing alpha particles at a sheet of thin gold foil. Detectors positioned around the gold foil detected the scattered alpha particles. It was found that while most of the alpha particles passed through undeflected or were scattered through very small angles, some were scattered through very large angles.
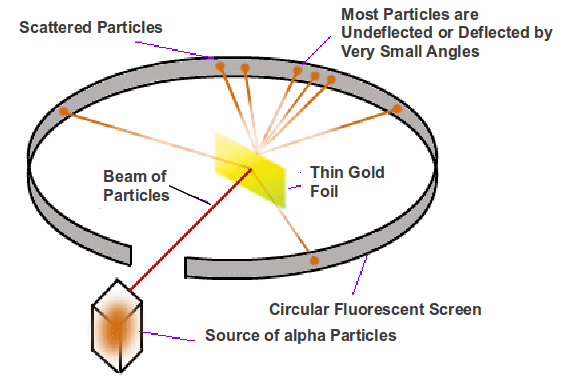
Rutherford showed that his results could be explained by modelling the atom as containing a very small core at the at the centre of the atom, containing all the positive charge. The electrons orbited the nuclus in a way similar to planets orbiting the Sun.
