We can find the specific latent heat capacity of a material by putting a mass![]() of that solid AT IT'S MELTING point into a mass
of that solid AT IT'S MELTING point into a mass![]() of the same substance in the liquid state as shown below.
of the same substance in the liquid state as shown below.
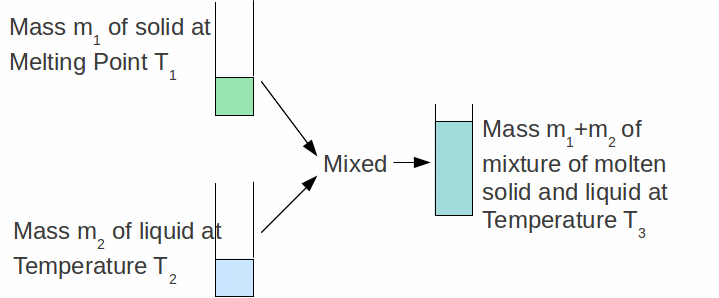
Provided we know the specific heat capacity of the substance in the liquid state, the specific latent heat of fusion can be calculate by assuming all heat lost by the liquid goes to melt the solid an raise it's temperature. The heat gained by the solid is used partly to melt it and partly to increase its temperature above the melting point:
![]()
![]()
These are equal so![]()
Example: 20g of ice at 0 degrees Celsius is put into 500ml of water at 25 degrees celsius. The temperature of the mixture after the ice has melted is 22.77 degrees Celsius. Find the specific latent heat of fusion of water.
![]()
