A simple cell is composed of two rods – electrodes - of different metals connected by a wire at one end. The other ends of the wire dip into an acid solution. Current flows through the wire as shown below – notice the direction of flow of the electrons, the actual charge carriers in the wire is in the opposite direction to the current.
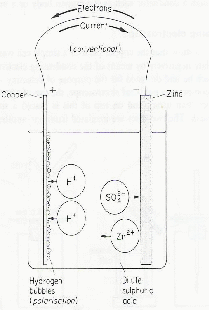
Most often copper and zinc are used as the electrodes. The zinc slowly dissolves into the sulphuric acid and bubbles of hydrogen are formed on the copper plate.
Pure sulphuric acid has the chemical symbol![]() but when the acid is made into a solution with water the
but when the acid is made into a solution with water the![]() group of atoms separates from the hydrogen atoms, taking two electrons with them, one from each hydrogen atom, leaving them both with a positive charge. The
group of atoms separates from the hydrogen atoms, taking two electrons with them, one from each hydrogen atom, leaving them both with a positive charge. The![]() group and the hydrogen are now ions. We can write down the equation
group and the hydrogen are now ions. We can write down the equation
![]()
As zinc dissolves from the zinc electrode the atoms go into solution in the form of zinc ions,![]() each zinc ion leaving behind two electrons on the electrode. These electrons are the source of the electric current which goes through the wire. We may think of the zinc ions being attracted into si=olution by the
each zinc ion leaving behind two electrons on the electrode. These electrons are the source of the electric current which goes through the wire. We may think of the zinc ions being attracted into si=olution by the![]() ions. At the same time, hydrogen ions move to the copper plate where the collect electrons and become a gas, forming bubble and leaving the solution.
ions. At the same time, hydrogen ions move to the copper plate where the collect electrons and become a gas, forming bubble and leaving the solution.
Normally when zinc dissolves in acid, internal molecular energy is produced and the solution gets warm. In the simple cell the action of the acid on the zinc results in the production of electrical energy instead.
