The Schrodinger equation for the hydogen atom takes the form![]()
This equation is separable which means that while the solution is a function of three variables, it is a product of three functions, each one of which is a function of only one variable, The general solution can be written![]() where
where![]() is itself a product of a function of
is itself a product of a function of![]() and
and![]() with a function of the form
with a function of the form![]() where
where![]() and
and![]() are integers. The function
are integers. The function![]() depends on
depends on![]() and
and![]() The first few functions of
The first few functions of![]() are given in the table below.
are given in the table below.
|
|
l |
|
|
1 |
0 |
|
|
2 |
0 |
|
|
2 |
1 |
|
|
3 |
0 |
|
|
3 |
1 |
|
|
3 |
2 |
|
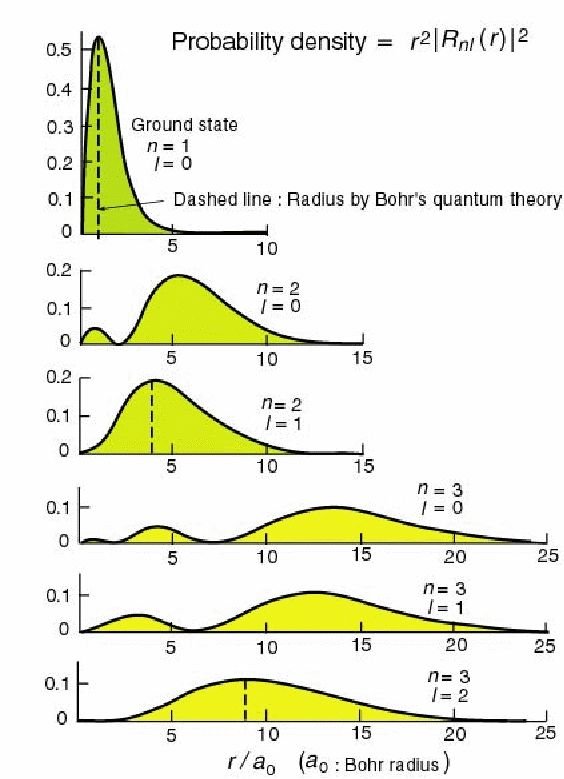
The probability of finding the electron at radius![]() is plotted below for these radial wavefunctions. Each radial wavefunction is normalized to 1.
is plotted below for these radial wavefunctions. Each radial wavefunction is normalized to 1.