Apparatus:
Photoelectric effect unit, 9V battery, 12V bulb, lab power supply set at 12V, black, rectangular cardboard tube, cathode ray oscilloscope, multimeter set at 20V DC, set of six filters, wires.
Background:
When a suitable metallic surface is illuminated with light of frequency f, photoelectrons are ejected from the surface with a range of kinetic energies. For the most energetic photoelectrons the Einstein equation gives:
![]()
where
![]() = energy of the incident light photon (h = Planck's constant, f = frequency of photon)
= energy of the incident light photon (h = Planck's constant, f = frequency of photon)
![]() = work function of the metal used
= work function of the metal used
![]() = kinetic energy of the most energetic photoelectron
= kinetic energy of the most energetic photoelectron
It is possible, therefore, to stop the emission by applying a suitable potential difference between the metal and an adjacent electrode. If this p.d. is V, is just large enough, then:
![]() where
where![]()
therefore![]() or
or![]()
where![]() and
and![]()
The above equation can be rearranged to give![]()
Dividing throughout by![]() gives
gives![]()
A graph of stopping voltage,![]() against
against![]() will have gradient equal to
will have gradient equal to![]()
The Experiment:
1. Connect the photoelectric unit to the 9V battery, cathode ray oscilloscope and multimeter as shown in diagram A opposite.
2. Connect the 12V light bulb to the ALTERNATING (YELLOW TERMINALS) output of the lab power supply set at 12V.
3. Remove the black plug from the 'amp input' on the photoelectric unit. (If it is present)
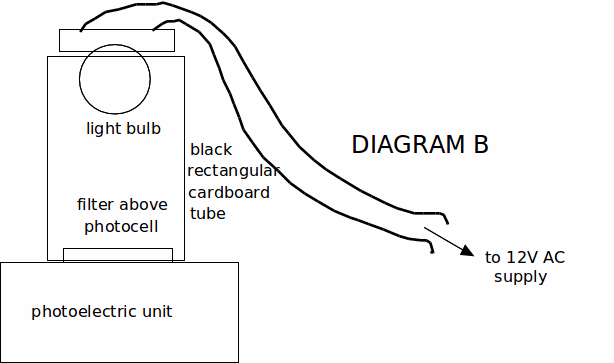
4. Place the violet filter (marked violet 380 - 450 nm) over the photocell, then place the cardboard rectangle and the light bulb above as shown in diagram B.
5. Rotate the dial on the photoelectric unit anticlockwise so that the multimeter reads zero.
6. Press down the red 'on' switch, and keep on pressing it, while adjusting the oscilloscope settings until you see a sinusoidal trace on the screen of amplitude of about 4cm.
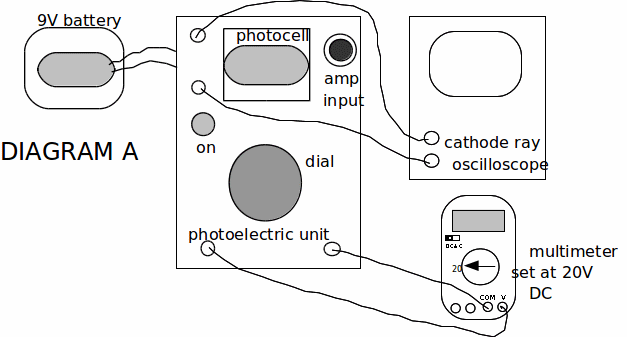
7. Now slowly turn the dial clockwise. This increases the reverse voltage to the photocell. Eventually this voltage will reduce the trace on the oscilloscope to zero. When this occurs note the reading on the multimeter which is a value for the stopping voltage, V for this situation.
8. Return the voltage to zero and repeat stage 7 two more times and so obtain an average value of V.
9. Note the minimum wavelength allowed by the filter,![]() (380nm for the violet filter)
(380nm for the violet filter)
10. Repeat stages 4 to 9 with the other five filters. Tabulate all of your measurements along with the calculation of (![]() ) for each filter.
) for each filter.
The other filters: blue (440 - 490nm); blue/green (480 - 530nm); green (530nm - 570nm)
orange (575 - 610nm); red (610 – 620nm). Note: nm = nanometres =![]()
11. Plot a graph of stopping voltage![]() against
against![]() measure its gradient and use it to obtain a value for Planck's constant
measure its gradient and use it to obtain a value for Planck's constant![]()
