Apparatus:
Paraffin oil, stop watch, top-pan balance to measure mass, insulated calorimeter consisting of an inner copper can, insulation, beaker, cover and electrical immersion heater, thermometer, ammeter & voltmeter, power supply set on 12V and wires.
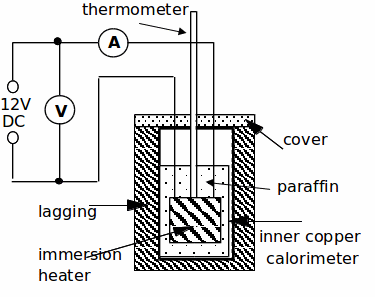
Procedure:
1. Measure the mass of the inner copper calorimeter.
2. Add paraffin to this can so that the can is approximately three-quarters full. Remeasure the mass and hence determine the mass of the paraffin.
3. Set up the apparatus as shown below.Set the ammeter on the 10 or 20A DC scale and the voltmeter on the 20V DC scale. DO NOT SWITCH ON THE POWER SUPPLY YET!
4. Wait for a few minutes so that the paraffin and calorimeter all reach the same initial temperature. Measure the initial temperature of the paraffin & copper calorimeter.
5. Start the stop watch and switch on the power supply. Immediately note the current and voltage readings.
6. KEEP STIRRING the paraffin and record its temperature every half minute until the temperature is about 20oC above the initial temperature. Try to read the thermometer more precisely than ±1oC.
7. Once the paraffin has risen in temperature by 20oC, switch off the power supply and note the time of heating, on the stop watch.. BUT KEEP ON READING THE THERMOMETER. Record the maximum temperature reached by the paraffin. This will probably occur about 3 or 4 minutes after you switch off the power supply.
8. Calculate the heat energy supplied by the heater using the equation
Heat energy supplied by the heater = Current x Voltage x Time
9. Calculate the temperature rise of both the paraffin and the inner calorimeter.
Temperature rise = Final maximum temperature - Initial temperature
10. Calculate the heat energy absorbed by the inner calorimeter using the equation:
Heat energy absorbed by calorimeter = Mass of calorimeter x SHC of calorimeter x Temperature rise
The SHC of the copper calorimeter is![]()
11. Calculate the heat energy absorbed by the paraffin from:
Heat energy absorbed by paraffin = Heat energy supplied by heater - Heat energy absorbed by calorimeter
12. Calculate the specific heat capacity of the paraffin from:
Heat energy absorbed by paraffin = Mass of paraffin x SHC of paraffin x Temperature rise
13. Suggest reasons why your answer above is likely to be too high.
14. Plot a graph of temperature against time. (Up to the time you switched off the heater).
15. Determine the maximum rate of rise of temperature by measuring the maximum gradient of this graph.
16. Use your value above to estimate the rate at which heat energy is being supplied to the paraffin and compare this with the power rating of the heater found by using the equation![]()
