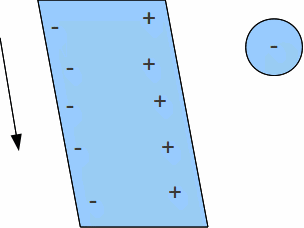
The molecules in polar liquids are like little dipoles. They have a positive end and a negative end, though each molecule as a whole is electrically neutral.
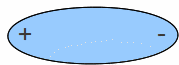
If an electric field is applied the molecules can line up to oppose the field, reducing the strength of it.
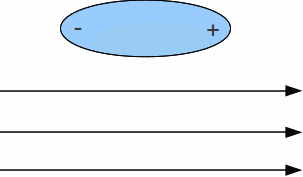
If a polar liquid is allowed to flow in the presence of a charge, the molecules of the liquid will align themselves so that one end is attracted to the charge and the liquid will bend towards the charge as it flows.