Surface tension causes a pressure difference between the inside of a soap bubble and the outside. We can imagine the bubble as consisting as two hemispherical surfaces. The bubble tends to contract so as to minimise the surface area of the bubble. As it contracts it compresses the air inside the bubble increasing the internal pressure until the pressure difference between the air inside and outside is balanced by the surface tension.
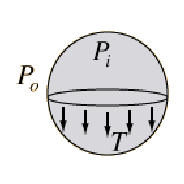
The forces on the upper hemisphere are due to the surface tension which acts at the boundary of the surface and the force due to the pressure difference between the air inside the bubble and outside.
We can write![]() where
where![]()
The 2 in the factor![]() arises because the bubble has an inside and an outside.
arises because the bubble has an inside and an outside.
Hence![]()
The smaller the radius of the bubble the larger the pressure difference.
For bubble in liquid there is only one surface hence![]() where
where![]()
Rearranging this equation gives![]() As the bubble rises the liquid and internal pressure become more equal, so the bubble expands.
As the bubble rises the liquid and internal pressure become more equal, so the bubble expands.
