The atoms in solids are largely fixed in position, able to move only by vibrating about a fixed position. That atoms are largely fixed in position in this way means that solids have a definite shape. The atoms inside a solid often have repeating, regular patterns.
The most regular structures are crystalline, divided into isotropic materials – having the same physical properties in any direction eg diamond – or anisotropic – having different physical properties in different directions eg carbon. The most common structures are shown below.
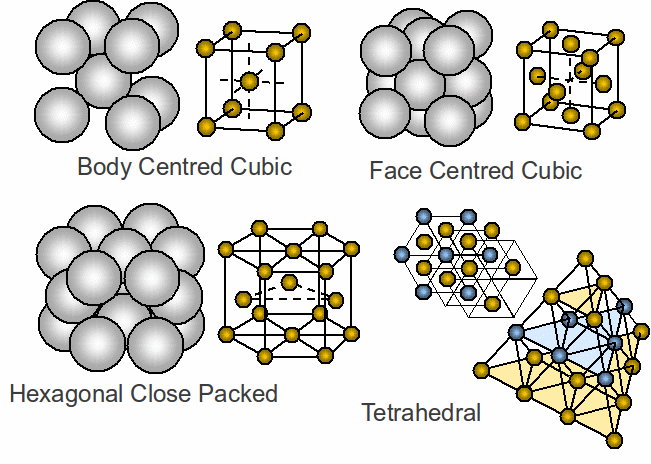
Face centred cubic and hexagonal close packing are most closely packed – 60% of metals have one of these structures.
Some examples of materials having each structure are given below:
Body Centred Cubic – potassium.
Face Centred Cubic – sodium chloride
Hexagonal Close Packing – zinc
Tetrahedral - silicon
