Most liquids contract when they solidify. The atoms in the liquid form bonds and move closer together.
If water at 0 degrees celsius is frozen – it becomes ice – then it expands and becomes less dense.
This is why ice floats – it is less dense than water. Pure water at 0 degrees Celsius has a density of![]() but pure ice at 0 degrees celsius has a density of
but pure ice at 0 degrees celsius has a density of![]()
Most liquids expand when they are heated. The molecules move faster, jostle each other more and are further apart on average. Water is strange in this respect.
If water at 0 degrees celsius is heated, it contracts and becomes less dense. It keeps contracting when heated until 4 degrees celsius, then it starts expanding.
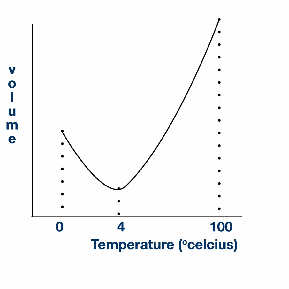
This is important for life on Earth. It means the bottom of a lake is the last part to freeze, so fish can usually survive the winter. This may have been very important during ice ages, or the supposed peropd in the Earth's history when it was almost completely frozen – only a ten mile stretch either side of the equator remaining liquid.
It will also have meant liquid would have flowed more readily. Rain would not have collected on the surface of ice. Being denser, it would have flowed off the ice and collected eventually in the ocean. This may have made the survival of life possible in the above mentioned period when the Earth was almost completely frozen.
