The atoms in diamond are all carbon. Each atom is joined to four other atoms. The atoms do not form planes, so there is no direction in which atoms can slip over each other.
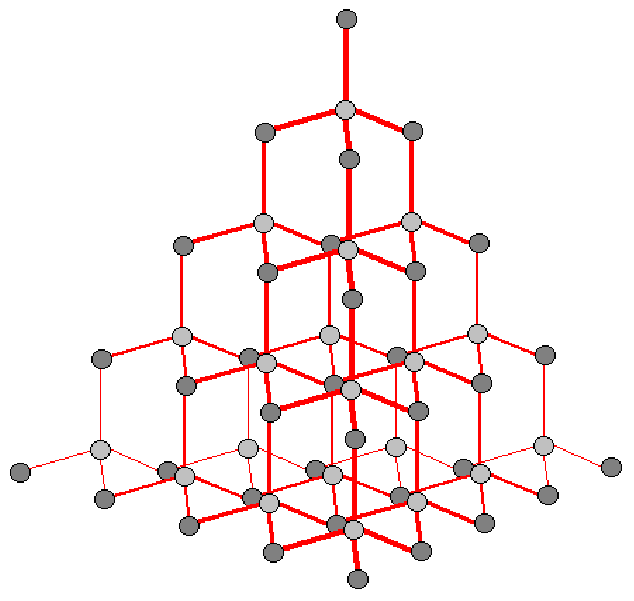
Taking this together with the fact that the bonds are strong means that diamond is a very hard material – the hardest known. It is used to cut other materials, including other diamonds. Diamond also has one of the highest melting points of any material because to melt diamond 4 bonds must be broken linking each carbon atom to four other atoms.
The molecular structure gives rise naturally to the 'diamond' shape.
