An isothermal expansion of a gas is such that the temperature of the gas is kept constant. The diagram below illustrates the isothermal expansion of a gas from![]() to
to![]() during which the pressure falls from
during which the pressure falls from![]() to
to![]()
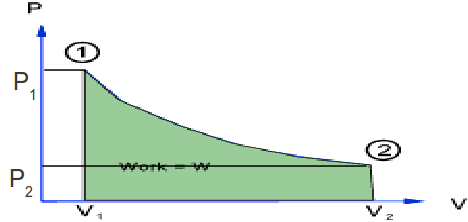
The gas expands during this expansion, so work is done by the gas. The amount of work done is equal to the area under the graph. We can write it as an integral:
![]()
If the gas is ideal then we can sue the ideal gas equation![]() rearranged as
rearranged as![]() to obtain the integral
to obtain the integral
![]()
We can evaluate this integral quite easily, obtaining![]() (1)
(1)
Because![]() the gas does work so W is positive.
the gas does work so W is positive.
Notice also that![]() for an isothermal process so that
for an isothermal process so that![]() This means we can write equation (1) as
This means we can write equation (1) as
![]()
