Maxwell showed using statistcal mechanics that the distribution of the speeds of the molecules of an ideal gas has a characteristic shape, shown in the graph below for![]() at several temperatures.
at several temperatures.
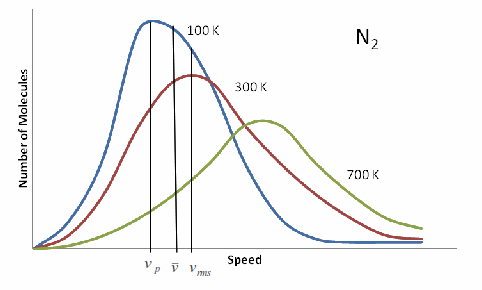
As the temperature increases the graph moves to the right in smooth way, keeping a characterisitc shape.
There are three important measures of molecular speed, shown on the graph above. These are
The most probable speed,![]()
The mean speed,![]()
The root mean square speed,![]()
The relationship between the three speed is given by the ratio![]() This ratio is
This ratio is![]() or 1: 1.13:1.23. This ratio is constant because it does not depend on temperature.
or 1: 1.13:1.23. This ratio is constant because it does not depend on temperature.
