In an ideal gas the molecules never collide since they are all assumed to have zero volume. In fact, obviously the molecules do have volume and collisions occur many times per second. We can estimate the average distance gas molecules travel between collisions. This distance is called the mean free path.
Consider![]() spherical molecules with diameter
spherical molecules with diameter![]() in a volume
in a volume![]() and suppose for simplicity that only one molecule is moving. The molecules collide whenever the distance between their centres is less than
and suppose for simplicity that only one molecule is moving. The molecules collide whenever the distance between their centres is less than![]() Draw a cylinder radius
Draw a cylinder radius![]() with axis parallel to the path of the molecule. The molecule collides with any other molecule whose centre is inside this cylinder. In a short time
with axis parallel to the path of the molecule. The molecule collides with any other molecule whose centre is inside this cylinder. In a short time![]() a molecule with speed
a molecule with speed![]() travels a distance
travels a distance![]()
|
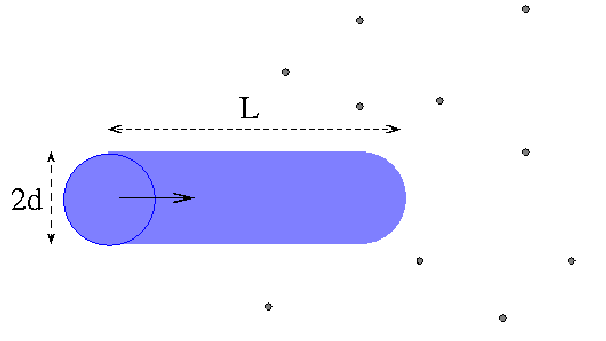
The volume of the cylinder is![]() There are
There are![]() molecules per unit volume so the number
molecules per unit volume so the number![]() with centres in the cylinder is
with centres in the cylinder is![]() so the number of collisions per unit time is
so the number of collisions per unit time is![]()
The average time between collisions is the reciprocal of this:![]() and the mean free path
and the mean free path![]()
Taking into account that all the molecules are moving introduces another factor of![]() so in fact
so in fact![]()
