The Carnot cycle represents the cycle of processes for a theoretical heat engine with the maximum possible efficiency. Such an idealised heat engine is called a Carnot engine.
It consists of an ideal gas undergoing the following processes.
-
Isothermal Expansion at
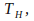 the temperature of the hot reservoir. An isothermal expansion takes place at constant temperature. Heat is allowed to enter the gas from the hot reservoir to keep the temperature constant.
the temperature of the hot reservoir. An isothermal expansion takes place at constant temperature. Heat is allowed to enter the gas from the hot reservoir to keep the temperature constant. -
Adiabatic expansion. No energy is allowed to enter or leave the gas.
-
Isothermal compression at
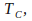 the temperature of the cold reservoir. An isothermal compression takes place at constant temperature. Heat is allowed to leave the gas to the cold reservoir to keep the temperature constant.
the temperature of the cold reservoir. An isothermal compression takes place at constant temperature. Heat is allowed to leave the gas to the cold reservoir to keep the temperature constant. -
Adiabatic compression. No energy is allowed to enter or leave the gas.
This cycle is illustrated below.
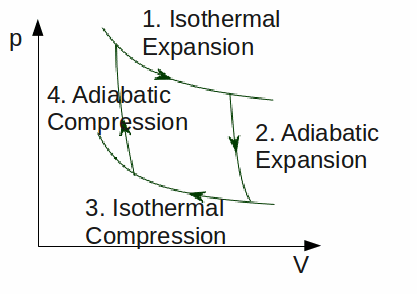
The temperatures of the hot and cold reservoirs determine the maximum efficiency of the engine.
![]() in Kelvins.
in Kelvins.
If![]() degrees Celsius = 293 Degrees Kelvin and
degrees Celsius = 293 Degrees Kelvin and![]() Degrees Celsius = 573 Degrees Celsius, then
Degrees Celsius = 573 Degrees Celsius, then![]()
