The protons in the nucleus are all positively charged. Like charges repel, so all the protons in the nucleus must be constantly repelling each other. In order for the nucleus to be stable, with all the protons confined within it, there must be another force acting. This force – called the strong nuclear force - must be
-
Strong. Gravity, though attractive, is far to weak, many powers of ten weaker than the force needed.
-
Short ranged. The force is active only inside the nucleus. We are familiar with the forces of electromagnetism and gravity since these are long ranged forces. If the strong nuclear force were also long range, it would overwhelm these two. If two nuclei are brought close, they will repel each other because of the electrostatic force. They must be brought very close together indeed for the strong force to dominate over the electrostatic force.
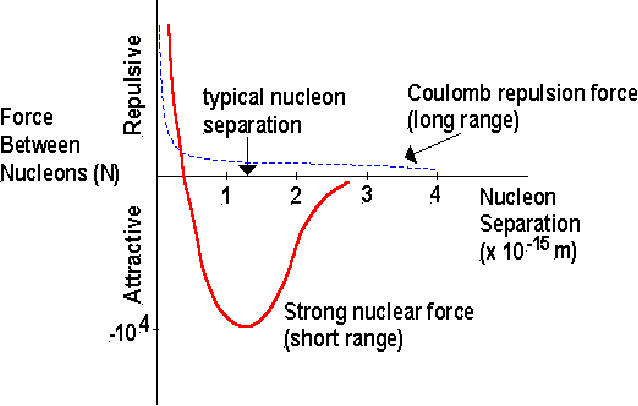
-
Felt by neutrons, since the protons are stably confined to the nucleus. In fact small nuclei tend to have equal numbers of neutrons and protons, and large nuclei tend to have more neutrons than protons.
In fact, the strong force can be repulsive for extremely short ranges, as shown above. There is an optimal distance, where the force between nucleons is most attractive, and the potential energy is minimum.
