There were once several competing models of the atom. The nuclear model pictured the atom as being like a mini solar system, with light electrons orbiting a heavy nucleus, while the plum pudding model pictured the atom as being a uniformly positively charged ball dotted with electrons.
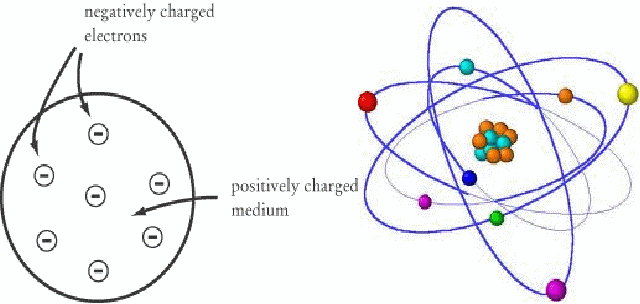
One of the most convincing pieces of evidence for the nuclear model of the atom is produced by the Geiger Marsden experiment. Positively charge alpha particles are fired at a thin sheet of gold leaf. Most of the alpha particles pass through through the gold leaf without noticeable deflection. A few are deflected through a small angle, but surprisingly, some are deflected through a very large angle.

A full mathematical analysis shows that the numbers being deflected at any angle agrees with an inverse square law of repulsion from the nucleus.
