The easiest way to measure the specific latent heat of vaporisation of a substance is to heat it to it's boiling point, and find the amount of electrical energy needed to boil away some amount of the substance as liquid.
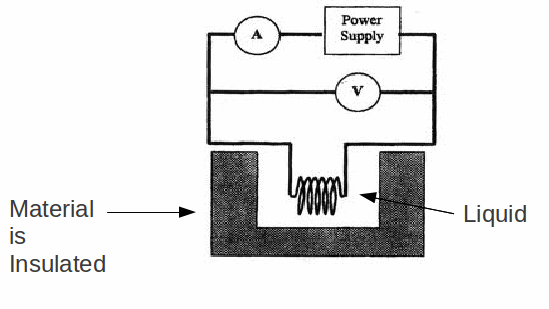
Equate the electrical energy supplied to the energy gained:![]()
I = current
V = power supply voltage
t = the time for which the power is on
m = mass of material evaporated
For example if 0.5000 kg of water is heated by a 240 W power supply supplying a 10A current for 1 hour and at the end of a minute there is only 0.4617 kg left then 0.5000-0.4617=0.0383 kg has evaporated.
![]()
In practice this is usually an overestimate, as energy losses are unavoidable. Perfect insulation is impossible, and the apparatus also will absorb some heat.
