The specific heat capacity c-1 of a substance can be found by mixing a known mass m-1 of that substance with a known mass m-2 of another substance of known specific heat capacity c-1 as shown in the diagram below.
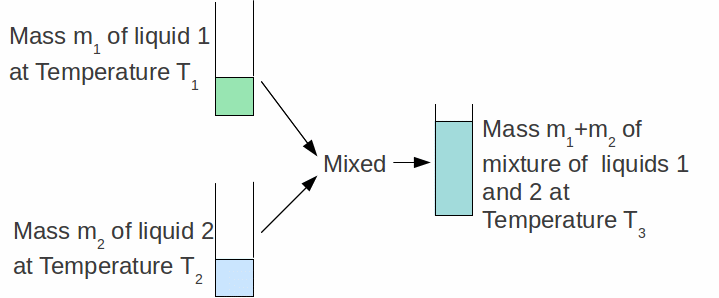
The initial temperature of the two substances are measured (in the case above the two substances are liquids). When they are mixed, they settle down to a third temperature![]() somewhere between
somewhere between![]() and
and![]()
If no energy is lost from the system then the heat lost from one liquid equals the heat gained by the other:
![]()
In fact energy is always lost from the system, because perfect insulation of the system is impossible, and because the containers also have heat capacities which this analysis does not take into account. A more sophisticated analysis would do so.
