Everybody knows that metals conduct electricity. If you apply a voltage, you get a current. Most elements are metals. However the are two other classes of materials – semiconductors and insulators. Which class a material falls into depends on how free the electrons are to move. In a metal many of the electrons are completely free. In the diagrams below, the electrons can be considered to occupied the green shaded regions, and the regions in which electrons can have enough energy to be able to move are coloured blue. This does not mean there are any electrons in the blue region. For there to be electrons free to move the blue and green regions have to overlap.
They do overlap for metals, hence metals can conduct electricity – the typical resistivity of a metal is![]() They don't overlap for semiconductors. Some energy may be given to the electrons so they can move from the valence band, where they normally are, to the conduction band, where they may conduct electricity. This energy may be in the form of heat or light. This implies that if you heat a semiconductor, it's resistance falls, since it can conduct electricity better. The typical resistivity of a semiconductor is
They don't overlap for semiconductors. Some energy may be given to the electrons so they can move from the valence band, where they normally are, to the conduction band, where they may conduct electricity. This energy may be in the form of heat or light. This implies that if you heat a semiconductor, it's resistance falls, since it can conduct electricity better. The typical resistivity of a semiconductor is![]()
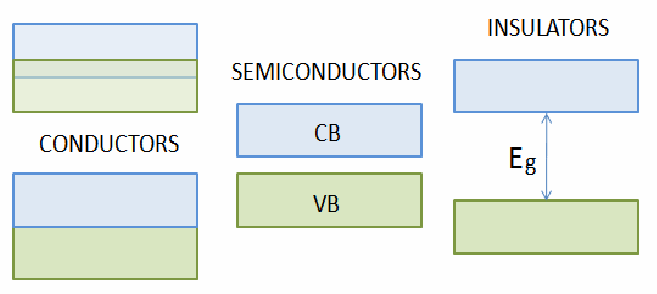
For insulators there is a much larger energy gap between the conduction and valence bands. The resistance of insulators is very high. A lot of energy must be given to the electrons to push them into the conduction band. The typical resistivity of an insulator is![]()
