The atomic model of the atom models the atom as a tiny, heavy nucleus surrounded by lighter electrons which orbit the nucleus in a way reminiscent of the planets orbiting the Sun.
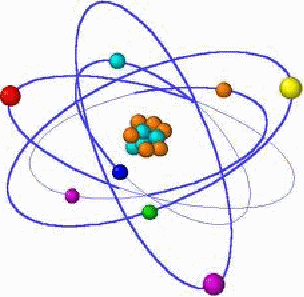
The nucleus is comprised of neutrons and protons, held together in the nucleus by the strong nuclear force. Each neutron or proton is about 1800 times heavier than an electron. Just as the name suggests, the neutron is electrically neutral. The proton is positively charged and the electron negatively charged, and the magnitude of both is the same at![]() The atom has the same number of protons as neutrons, and is therefore electrically neutral. The number of protons determines which element the atom is, while the number of neutrons may vary. Two atoms with the same number of protons but different numbers of neutrons are different isotopes of the same element and have the same chemical properties, but not the the same physical properties. They have different masses for example.
The atom has the same number of protons as neutrons, and is therefore electrically neutral. The number of protons determines which element the atom is, while the number of neutrons may vary. Two atoms with the same number of protons but different numbers of neutrons are different isotopes of the same element and have the same chemical properties, but not the the same physical properties. They have different masses for example.
The orbits of the electrons around the nucleus in fixed. Being of opposite charges, electrons and protons attract each other, necessary for a stable orbit. Each orbit is associated with a specific energy, and an electron may move between different orbits by emitting or absorbing a specific amount of energy.
The atom as a whole has a diameter of around![]() and the diameter of the nucleus is
and the diameter of the nucleus is![]() The volume of the nucleus is therefore of the order of
The volume of the nucleus is therefore of the order of![]() of the volume of the atom.
of the volume of the atom.
