The saturated vapour pressure of a liquid can be determined using the apparatus below. The air is first pumped out using a vacuum pump. The liquid is then boiled and evaporates. Some of the vapour fills the apparatus and some condenses. The water is then allowed to cool to a set temperature. At this temperature the water will exert a satuarted vapour pressure![]()
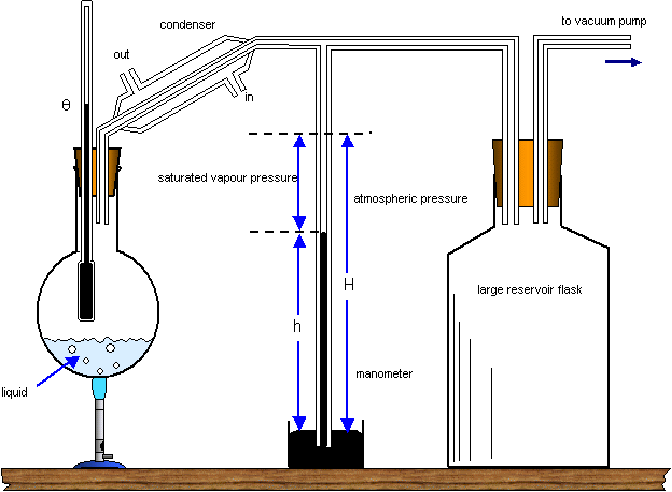
If the atmospheric pressure is![]() and the liquid in the barometer column (mercury) has density
and the liquid in the barometer column (mercury) has density![]() then
then![]() so
so![]()
The air pressure within the apparatus can be varied using the cauum pump and the saturated vapour pressure of water can so be found for any air pressure.
