The boiling temperature of water can be increased by increasing the pressure above the water. To vaporize in the pressurized container, the water molecules need to increase their kinetic energy. This increase in kinetic energy translates into a higher boiling temperature. Conversely, with a reduced pressure, the water molecules need less kinetic energy to vaporize than they do in an open container. This decrease in kinetic energy translates in a lower boiling point.
Whether water exists as a solid, liquid, or gas depends on its temperature and the pressure of the surrounding environment. Change the temperature or pressure, and water may undergo a phase change. We are familiar with how water responds to changes in temperature – at sea level, it typically freezes at 0 degrees Celsius and boils at 100 degrees Celsius. For an understanding of the effects of pressure we need to look at the following phase change diagram
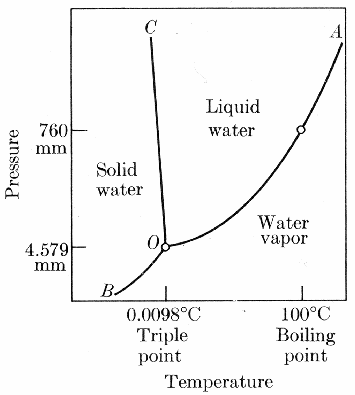
Remember that water is in equilibrium with ice when it freezes at the same rate that the ice melts and it is in equilibrium with vapour when it evaporates at the same rate that the vapour condenses.
Every substance has its own unique phase diagram. At a boundary line between two phases, two phases are in equilibrium with one another – that is, the rate of molecules leaving a phase equals the number returning. We refer to the inclination of a molecule to change phase and establish an equilibrium as its vapour pressure. Vapour pressure increases with temperatures. At higher temperatures, a particle’s kinetic energy is higher, and, with more energy available at higher temperatures, it is easier for particles to change phase. Even solids such as ice have a vapour pressure and can sublimate directly to the vapour phase.
If two phases are not in equilibrium, molecules will change from one phase to the other until an equilibrium is established. The water evaporating out of a lake in the desert is trying to establish an equilibrium with the dry desert air. In the case of a puddle, the water disappears completely before an equilibrium is established. Each temperature-pressure combination has its own equilibrium point. If you connect these many equilibrium points, you will have drawn the boundary lines on the phase diagram.
All three boundary lines meet at a point called the triple point. At this temperature and pressure, all three phases are in equilibrium with one another. In other words, at the triple point, vapour sublimates to ice and condenses to liquid, liquid evaporates to vapour and freezes to ice, and ice melts to liquid and sublimates to vapour all the same rate. A minuscule change in either temperature or pressure will move the phase changes away from the triple point. Away from the boundary lines, water exists in a single phase over a particular range of temperatures and pressures.
The phase diagram above is the key to understanding why we have not seen liquid water on Mars.
Below 6.1 millibars, liquid water cannot exist, irrespective of the temperature. Water's vapour pressure is just too high to remain a liquid below this level. When atmospheric pressure falls below 6.1millibars, water can only exist as ice or vapour, depending on the temperature. The atmospheric pressure at the Martian surface hovers just above 6.1 millibars. Any water that might form on a warm afternoon from melting ice would quickly disappear. If the vapour pressure of the warmed water exceeded atmospheric pressure, it would boil. If, instead, its vapour pressure stayed below atmospheric pressure, the water would evaporate. The temperature and pressure combinations on Mars make liquid water theoretically possible on an occasional basis, but the long term presence of water is impossible.